New Technology Makes Cancer Tumors Eliminate Themselves
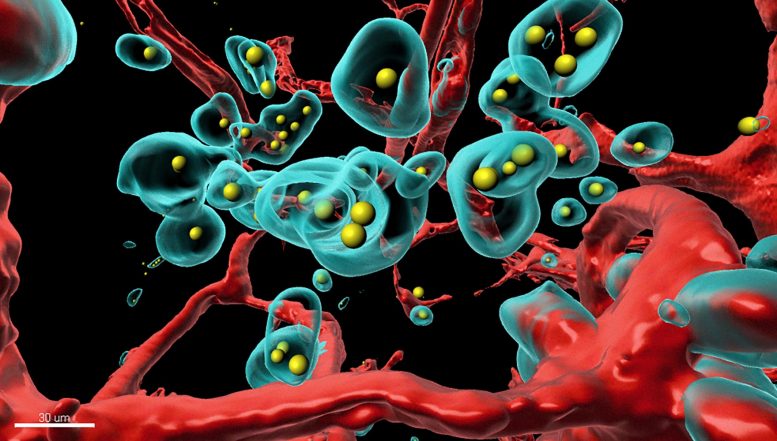
A piece of the tumor was made completely transparent and scanned in 3D with a special microscope. The components labeled with fluorescent colors were rendered in a rotatable 3D representation on the computer (red: blood vessels, turquoise: tumor cells, yellow: therapeutic antibody). Credit: Plückthun Lab
A new technology developed by University of Zurich researchers enables the body to produce therapeutic agents on demand at the exact location where they are needed. The innovation could reduce the side effects of cancer therapy and may hold the solution to better delivery of Covid-related therapies directly to the lungs.
Scientists at the University of Zurich have modified a common respiratory virus, called adenovirus, to act like a Trojan horse to deliver genes for cancer therapeutics directly into tumor cells. Unlike chemotherapy or radiotherapy, this approach does no harm to normal healthy cells. Once inside tumor cells, the delivered genes serve as a blueprint for therapeutic antibodies, cytokines and other signaling substances, which are produced by the cancer cells themselves and act to eliminate tumors from the inside out.
Sneaking adenoviruses past the immune system undetected
“We trick the tumor into eliminating itself through the production of anti-cancer agents by its own cells,” says postdoctoral fellow Sheena Smith, who led the development of the delivery approach. Research group leader Andreas Plueckthun explains: “The therapeutic agents, such as therapeutic antibodies or signaling substances, mostly stay at the place in the body where they’re needed instead of spreading throughout the bloodstream where they can damage healthy organs and tissues.”
The UZH researchers call their technology SHREAD: for SHielded, REtargetted ADenovirus. It builds on key technologies previously engineered by the Plueckthun team, including to direct adenoviruses to specified parts of the body to hide them from the immune system.
High amount of drugs in the tumor, low concentration in other tissues
With the SHREAD system, the scientists made the tumor itself produce a clinically approved breast cancer antibody, called trastuzumab, in the mammary of a mouse. They found that, after a few days, SHREAD produced more of the antibody in the tumor than when the drug was injected directly. Moreover, the concentration in the bloodstream and in other tissues where side effects could occur were significantly lower with SHREAD. The scientists used a very sophisticated, high-resolution 3D imaging method and tissues rendered totally transparent to show how the therapeutic antibody, produced in the body, creates pores in blood vessels of the tumor and destroys tumor cells, and thus treats it from the inside.
Use to combat Covid-19 being investigated
Plueckthun, Smith and colleagues emphasize that SHREAD is applicable not only for the fight against breast cancer. As healthy tissues no longer come into contact with significant levels of the therapeutic agent, it is also applicable for delivery of a wide range of so-called biologics – powerful protein-based drugs that would otherwise be too toxic.
In fact, members of the Plueckthun group are currently applying their technology in a project aimed as a therapy for Covid-19. Adenoviral vectors are already being used in several of the COVID vaccines, including the Johnson & Johnson, AstraZeneca, China’s CanSino Biologics and Russia’s Sputnik V vaccines – but without the innovative SHREAD technology. “By delivering the SHREAD treatment to patients via an inhaled aerosol, our approach could allow targeted production of Covid antibody therapies in lung cells, where they are needed most,” Smith explains. “This would reduce costs, increase accessibility of Covid therapies and also improve vaccine delivery with the inhalation approach.”
Reference: “The SHREAD gene therapy platform for paracrine delivery improves tumor localization and intratumoral effects of a clinical antibody” by Sheena N. Smith, Rajib Schubert, Branko Simic, Dominik Brücher, Markus Schmid, Niels Kirk, Patrick C. Freitag, Viviana Gradinaru and Andreas Plückthun, 17 May 2021, Proceedings of the National Academy of Sciences.
DOI: 10.1073/pnas.2017925118
Funding: Swiss National Science Foundation, NIH/National Cancer Institute
